
Introducing the NITRO Family
In the race to achieve interbody fusion, material matters.
Unveiling Advanced Spine Solutions
Explore the NITRO Series
Discover the precision-engineered NITRO series, crafted from advanced silicon nitride to enhance patient outcomes. These implants leverage exceptional properties to provide superior technological advancements in patient care.
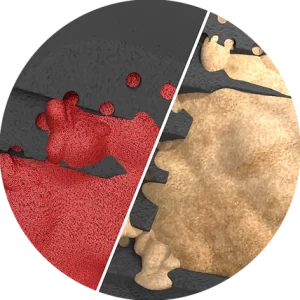
NITRO implants have demonstrated superior protein absorption and increased osseointegration compared to other biomaterials.

Silicon nitride possesses unique bacteriostatic properties, inhibiting the growth of bacteria.
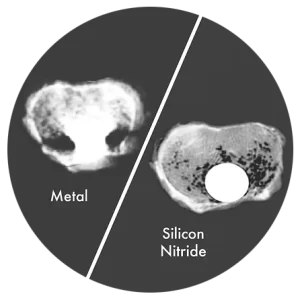
The biomaterial used in NITRO allows for artifact-free CT Scan imaging, enabling clear visualization of the implant and surrounding tissues.

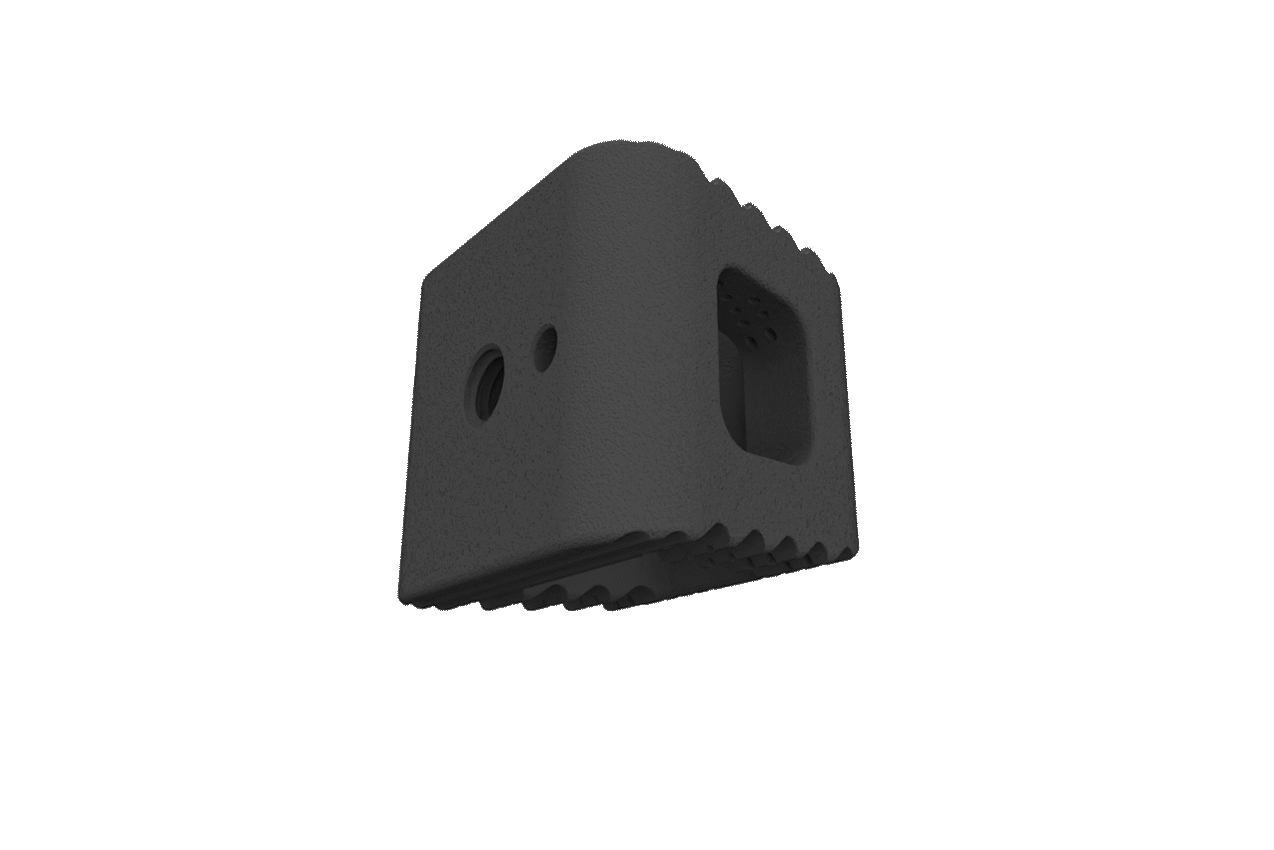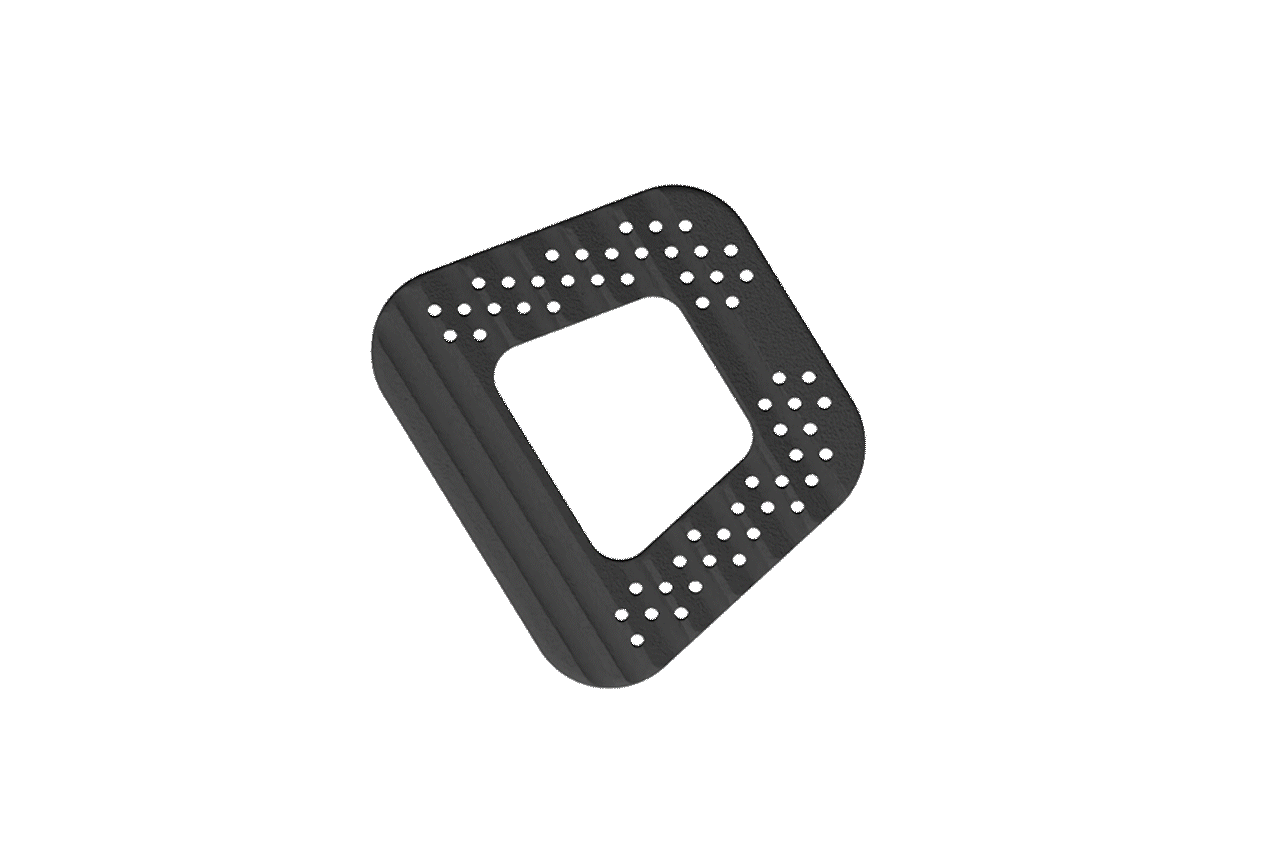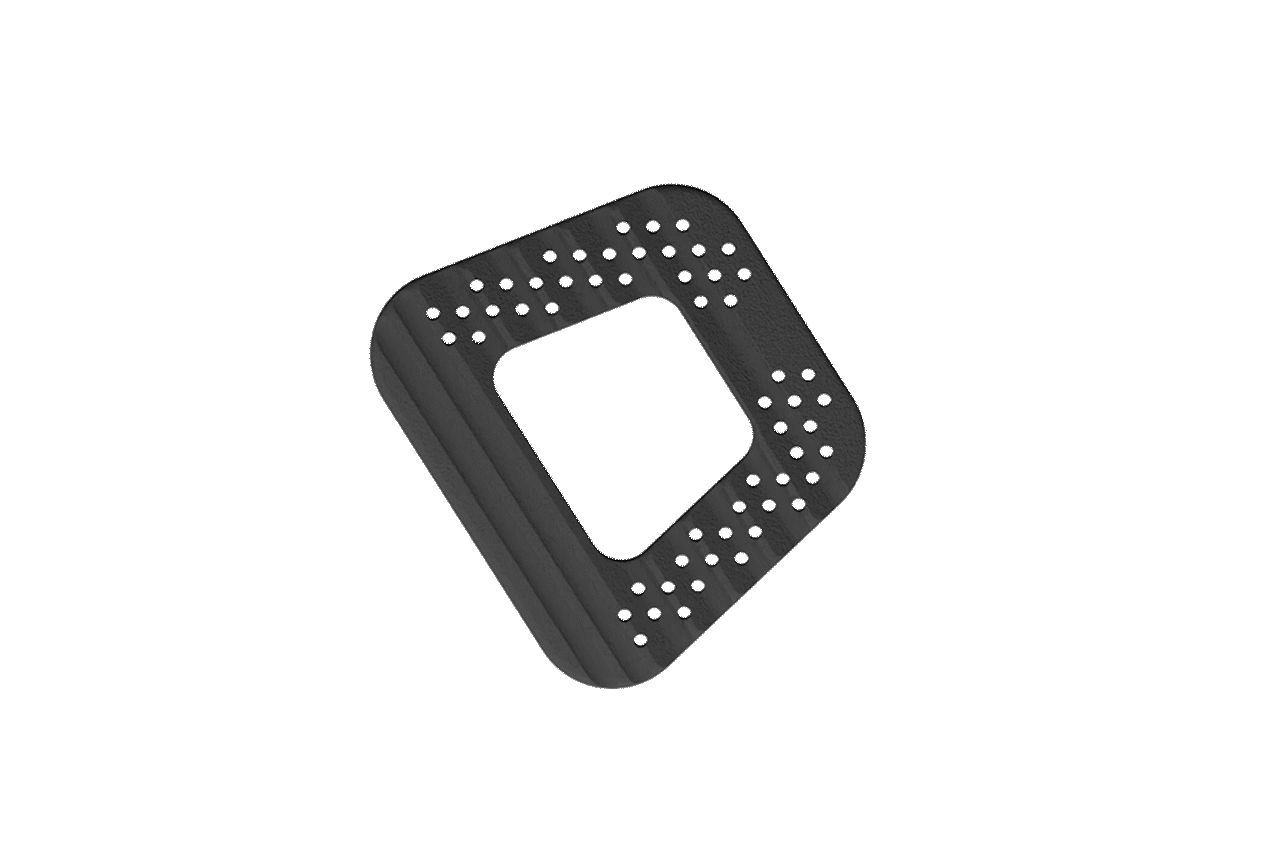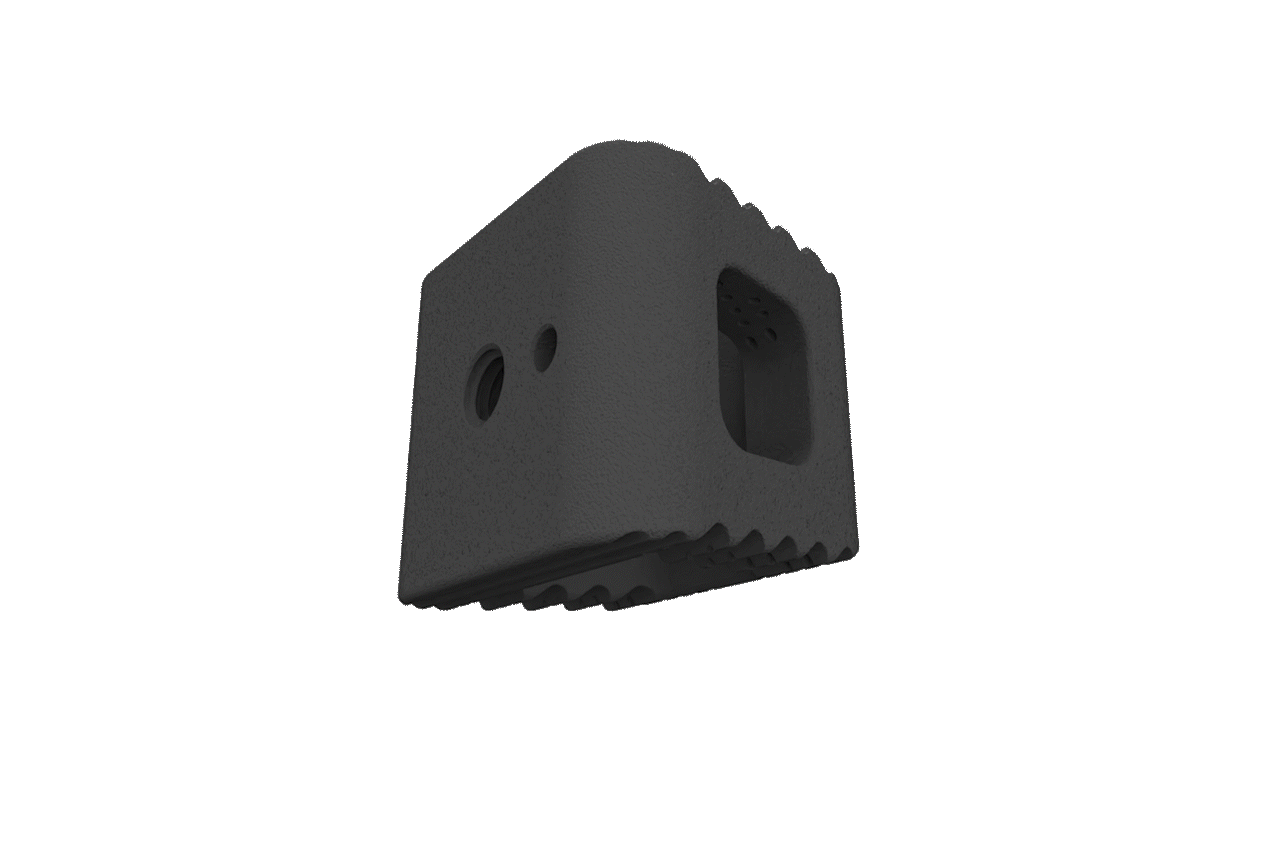
The NITRO interbody fusion family incorporates precisely sized axial pores that promote capillary action and a pathway for bony through-growth.
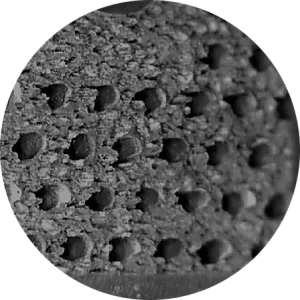
Macro-texturing provides even greater surface area throughout the implant, increasing bony contact and enhancing innate bacteriostatic properties.

The NITRO interbody fusion family offers a robust and comprehensive variety of standalone integration options, lordotic offerings, and size configurations
Note: Standalone integration is pending market launch.
Discover the Power of NITRO
Enhancing Spinal Health with Advanced NITRO Implants
Next-generation NITRO implants harness all the innate advantages of silicon nitride, in combination with innovative enhancements for an even greater osteogenic and bacteriostatic response.
Experience the Future of Spine Care
Request a Demo of Our NITRO Implants
Discover firsthand the superior performance and benefits of NITRO implants. Schedule a demo today and see why leading surgeons are choosing our advanced solutions for their patients.


